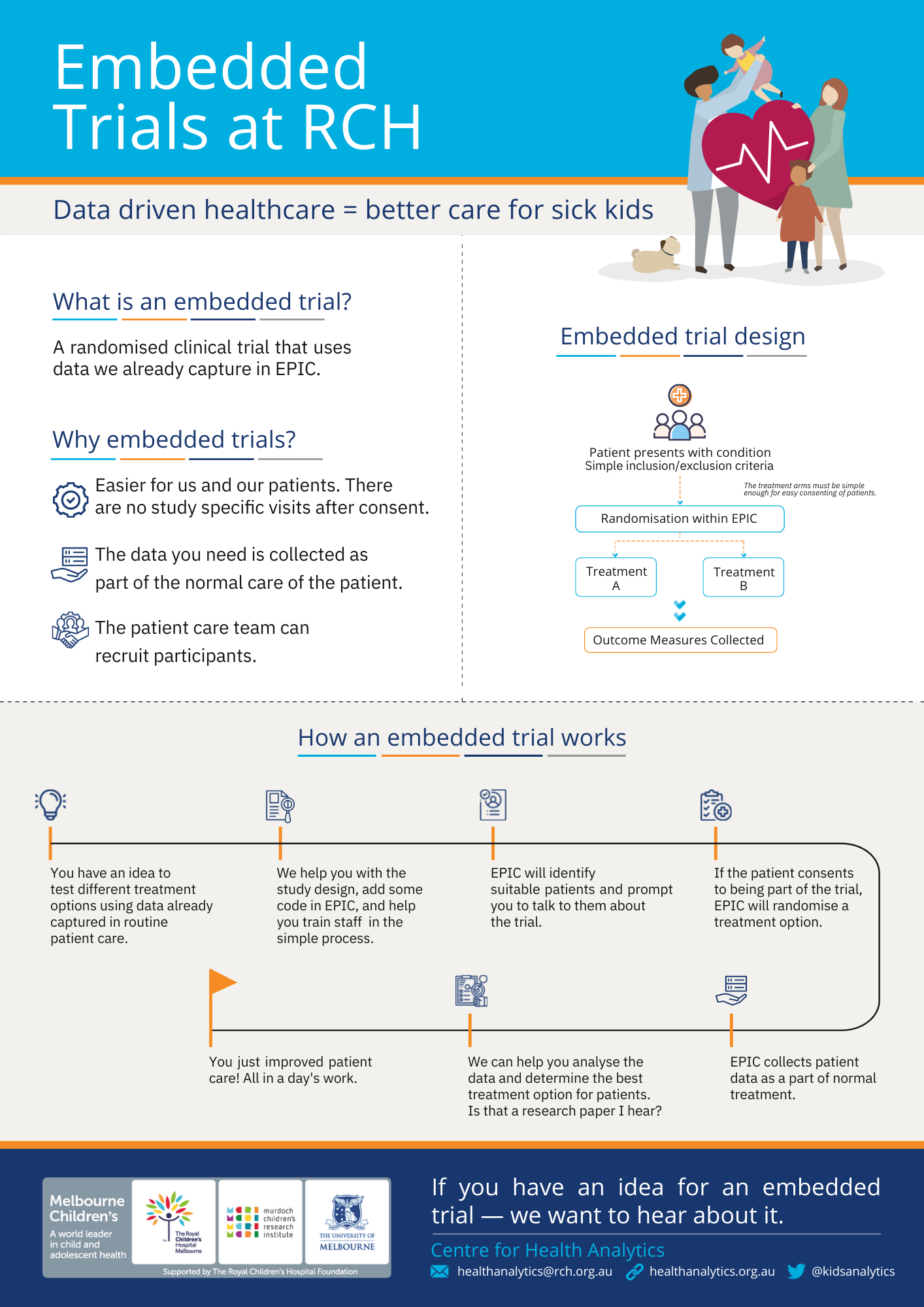
Embedded Clinical Trials
At least half of our children are prescribed at least one medicine each year. But there is often little data on the safety, effectiveness and dosage of these treatments because they are verified by adult clinical trials. We are able to compare paediatric treatment options safely and effectively using embedded trials.
An embedded trial tests different treatment options using randomisation and data that is already captured in EPIC as part of routine patient care.
Currently, we are running the Early versus Late Stopping of Antibiotics in Children with Cancer and High-risk Febrile Neutropenia (ELSA-FN) trial and the POPCORN trial at RCH with other embedded trials in the works.